N L Ml Ms Chart
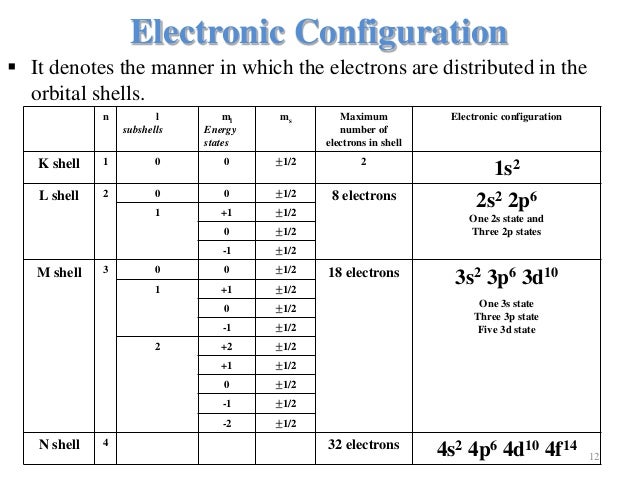
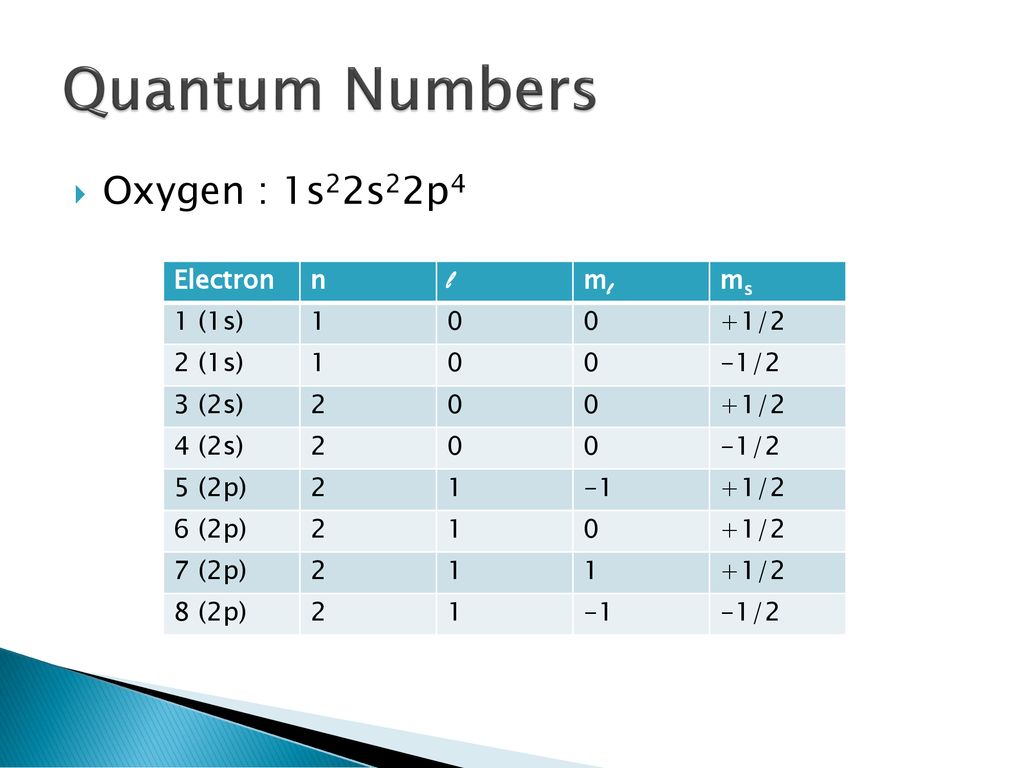
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is the electron configuration of an blank atom.
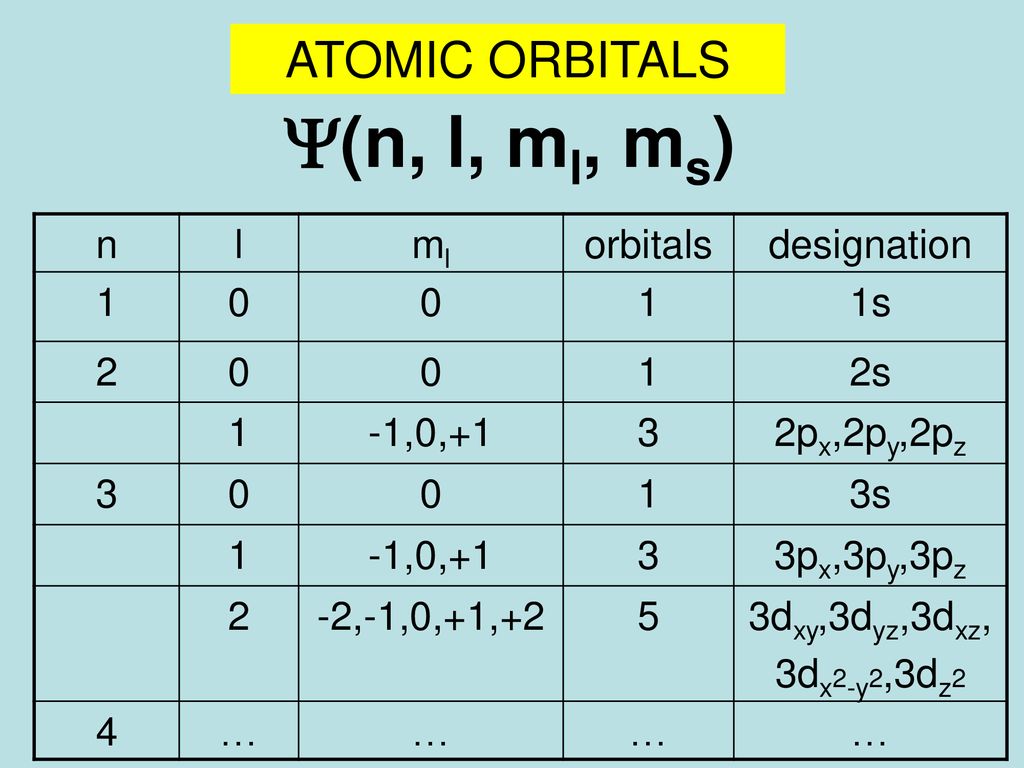
N l ml ms chart. The three quantum numbers n l and m that describe an orbital are integers. 1 m2 1 2. Then n minus one would be equal to one.
V n4 l0 ml 0 ms 12 n4 l0 ml 0 ms -12 c. Ml 2l 1. L is equal to zero.
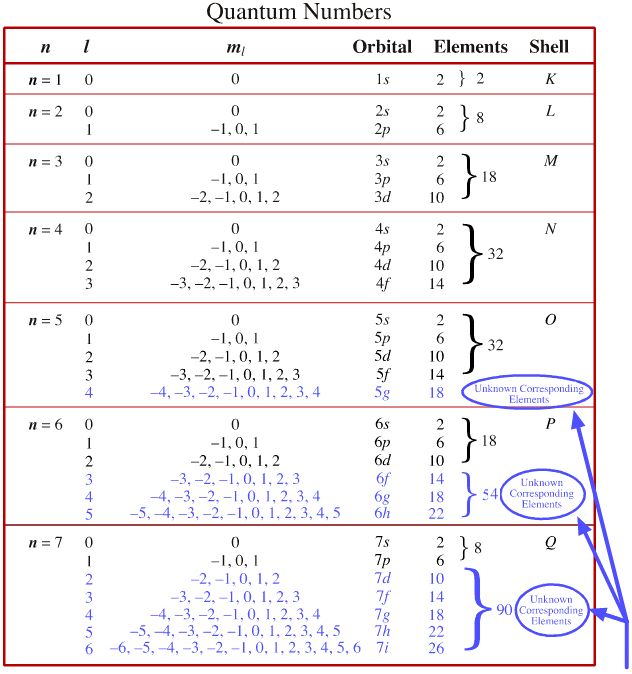
First - Primary Quantum number n size of electron cloud n 1 up to in reality n 1 -7 Second Azimuthal or Angular Momentum Quantum number l shape of electron cloud. Use this page to learn how to convert between milliliters and liters. MLsLmin 1 Lmin 16666666666667 mLs mLsLs 1 Ls 1000 mLs mLsmLmin 1 mLs 60 mLmin mLsgal USd 1 mLs 22824465 gal USd mLsgal USh 1 gal USh 10515032733215 mLs mLsgal USmin 1 gal USmin 63090196405926 mLs mLsgal USs 1 gal USs 37854117834003 mLs mLsgal UKd 1 mLs 19005343.
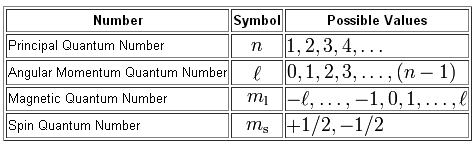
So for example if. B n 3 l 2 m l 1 this is allowable l is less than n and m l can be any of 2 -1 0 1 2 c n 2 l 0 m l 0 this is allowable l is less than n and m l can only be 0 d n 5 l 3 ml -4 this is not allowable. The principal quantum number n cannot be zero.
All orbitals that have the same value of n. It is either positive or negative one half. 2 Azimuthal quantum number l 1.
So we have two possible values for l. N5 l 0because it is a s orbital ml 0because l 0 so it has got no z-component of l ms - 12 So assuming first one was 5s1 then the second electron is the last remaining electron that can fit into 5s1 ie maximum filled at 5s2s orbitals can only hold max 2 electrons. Type in your own numbers in the form to convert the units.
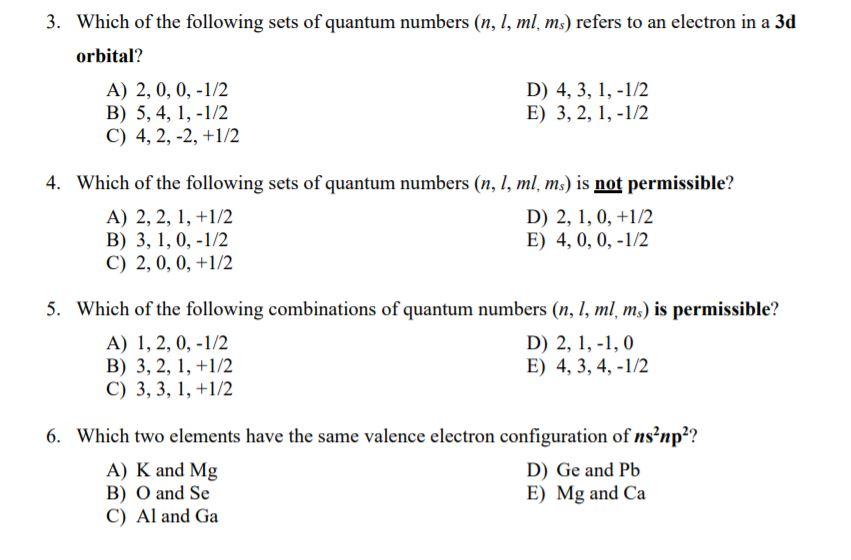
N 4 l 1 m. The angular quantum number l can be any integer between 0 and n - 1. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital.
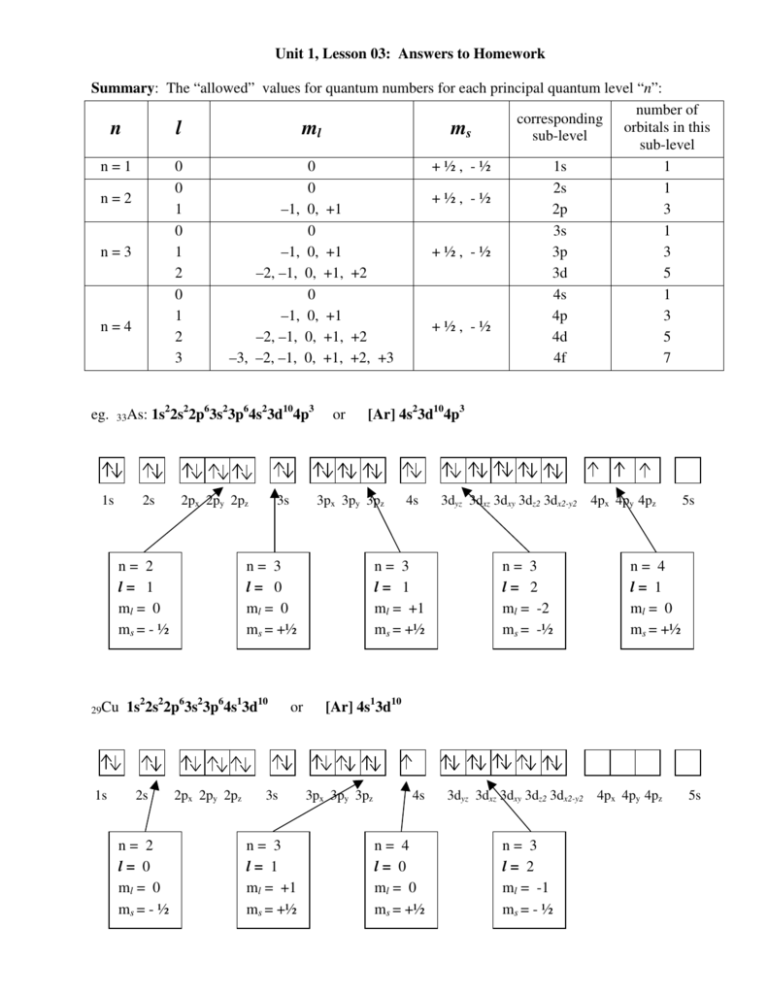
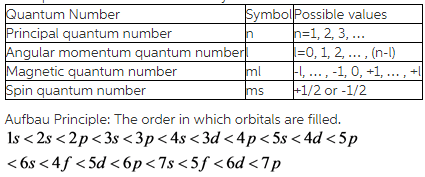
N 1 2 3 8. L 0 up to n-1. Ms is spin quantum number and refers to spin of electron.
Cu n4 l0 ml 0 ms 12 n4 l0 ml 0 ms -12 e. 1 ml to l 0001 l. 0 1 2 3 and so on.
On page 138 answer questions 2 3 5 6. 50 ml to l 005 l. In that case the value for n is 3 because it is a 3p electron l is 1 because it is a p electron ml is 1 the -1 0 and 1 are essentially degenerate and at the same energy level but convention places the 1 last and ms is 12 because the p orbital is doubly filled with both spin values -12 and 12.
10 ml to l 001 l. That is no two electrons can have the same values for n l ml and ms. Note that rounding errors may occur so always check the results.
Quick conversion chart of ml to l. L could be equal to zero and l could be equal to one. Br n4 l0 ml 0 ms 12 n4 l0 ml 0 ms -12 n4 l1 ml -1 ms 12 n4 l1 ml 0 ms 12 n4 l1 ml 1 ms 12 n4 l1 ml -1 ms -12.
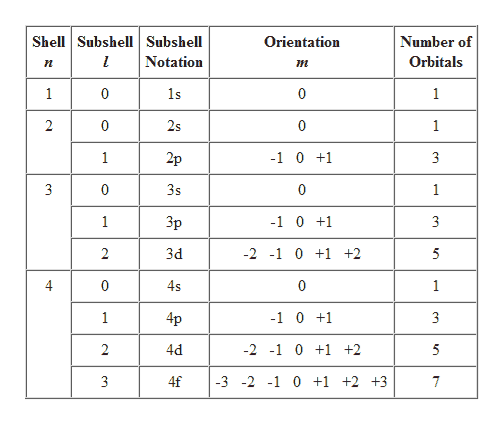
Determine n and l by identifying the period and the block that the element is located in. Determine ml by labeling the block from -l to l. M l can only have values 3 2 -1 0 1 2 3 3.
Ml is magnetic quantum number and refers to the number of orbitals per subshell. Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. Principal Quantum Number n.
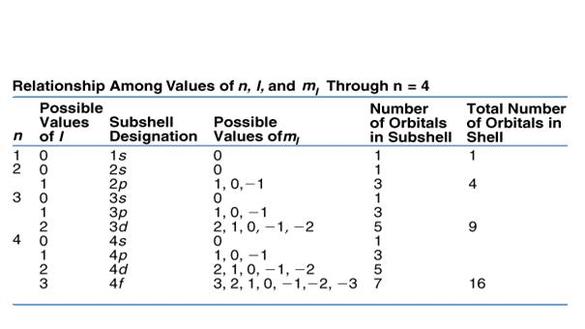
3 Magnetic quantum number ml 101. Notice that the number of allowed values for l is equal to n. If n is equal to two what are the allowed values for l.
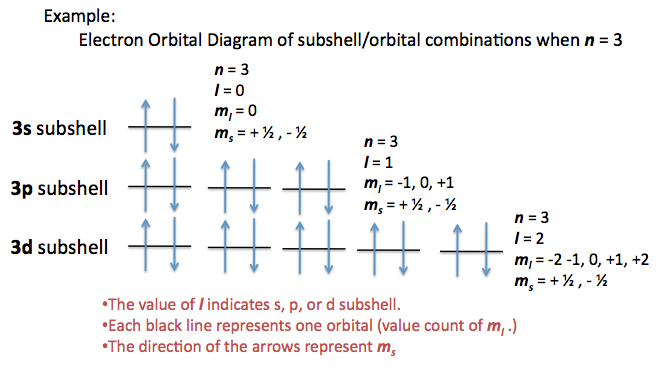
State the four quantum numbers and the possible values they may have. 1 Principal quantum number n 3. If n 3 for example l can be either 0 1 or 2.
Arsenic at maximum an f-subshell can hold blank electrons a d-subshell can hold blank electrons and a p-subshell can hold blank electrons. Ni n4 l0 ml 0 ms 12 n4 l0 ml 0 ms -12 d. When l 0 s cloud 1 p cloud 2 d cloud 3 f.
1 m2 1 2. These orbitals are called degenerate or equal. N1 l 0 ml 0 ms-12 b.
L is angular momentum quantum number. Ms 1 2 a spin-up electron. N is equal to two.
It is the number of subshells per principal energy level. L goes zero one and so on all the way up to n minus one. Determine ms by determining if.
4Spin quantum number s 1212. Lets do the next shell. 1 cubic meter is equal to 1000000 ml or 1000 l.
The allowed values of n are therefore 1 2 3 4 and so on. N 4 l 1 m. Although the first three quantum numbers identify a specific orbital and may have the same values the fourth is significant and must have opposite spins.
N l ml ms.