Gibbs Free Energy Spontaneous Chart
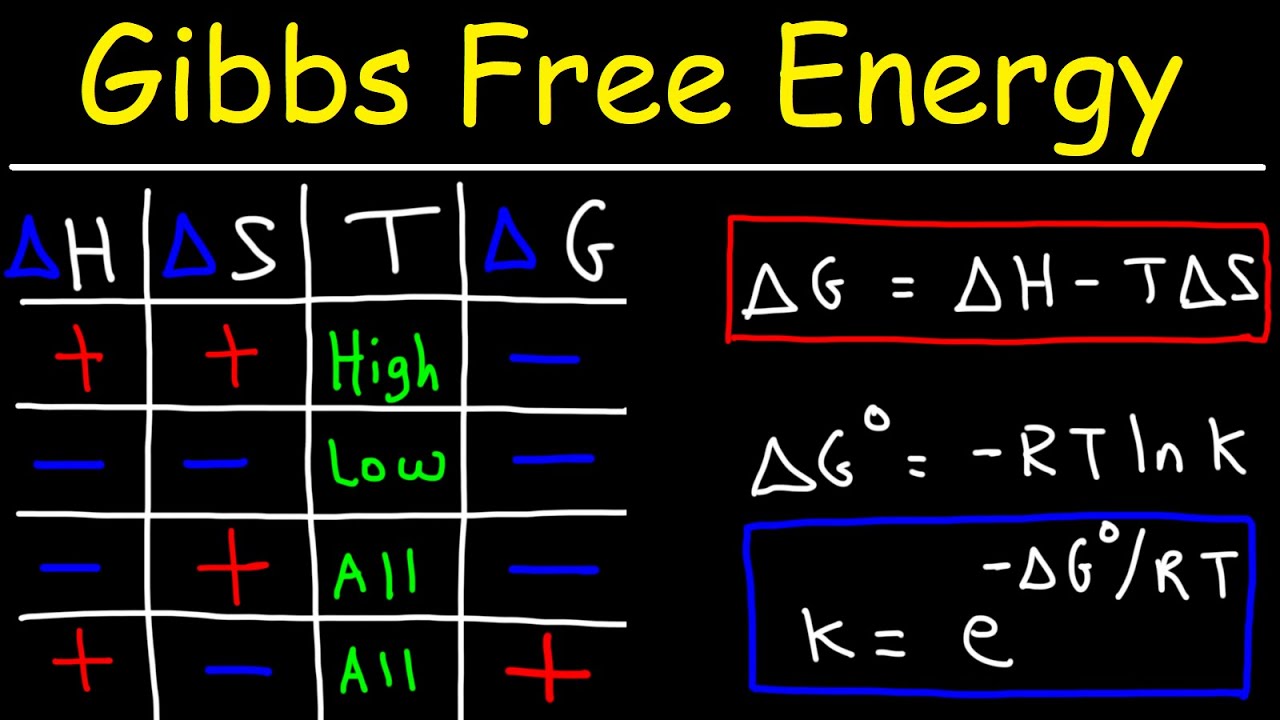
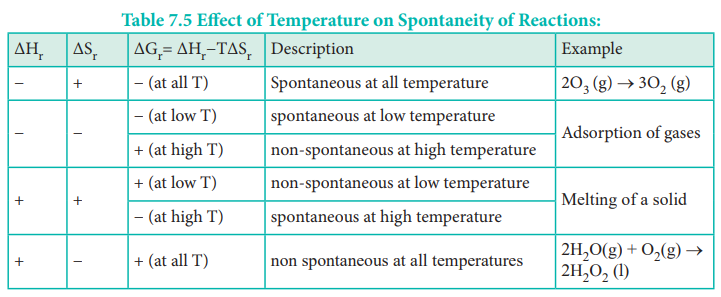
The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.
Gibbs free energy spontaneous chart. In the article Gibbs free energy formula you will learn the meaning of Gibbs free energy standard Gibbs free energy change its unit derivation of Gibbs- Helmholtz equation ie Δ G Δ H T Δ S conditions of spontaneity the relation between free energy and equilibrium constant and many more. Where is enthalpy is temperature in kelvin and is the entropy. The free energy change D G is equal to -T D S univ and it applies just to a system itself without regard for the surroundings.
Here ΔG 0. G H - TS The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions. The Gibbs-Helmholtz Equation Frequently we wish to run reactions at temperatures other than 25oC.
Just like thermochemical equations reactions w associated free energies can be manipulated with the same rules applying. Gibbs Free Energy kJmol NH 4 2O l -43070096 26752496 -26710656 NH 4 2SiF 6 s hexagonal -268169296 28024432 -236554992 NH 4 2SO 4 s -118085032 2200784 -90190304 Ag s 0 4255128 0 Ag g 28455384 172887064 24568448 Ag1 aq 105579056 7267608 77123672 Ag 2 g 40999016 25702312 358778 Ag 2C 2O 4 s -6732056 2092. The table below shows the standard enthalpy of formation the standard Gibbs free energy of formation standard entropy and molar heat capacity at constant pressure of several inorganic compounds.
If Gibbs Free Energy is ever negative or less than 0 then its a spontaneous reaction. 211 rows Chem Table Gibbs Free Energy of Formation Delta G May 2nd. Therefore the Gibbs free energy symbol ie.
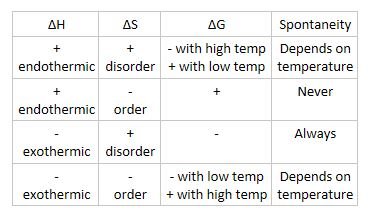
Gibbs free energy is an excellent indicator of if we have a spontaneous reaction or a non-spontaneous reaction. Gibbs Free Energy Change G Gibbs free energy is a term that combines the effect of enthalpy and entropy into one number The balance between entropy and enthalpy determines the feasibility of a reaction. When ATP reacts with water hydrolysis energy is released.
The formula for Gibbs Free Energy is written in terms of ΔH and ΔS along with temperature. It is easy as long as you remember to convert the entropy change value into kJ. 2ggiven that the standard free energies of formation for CO.
See also Standard enthalpy of formation Gibbs free energy of formation entropy and molar heat capacity of organic substances and Thermodyamics key values internationally. So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in. Otherwise it is non-spontaneous as it requires a continuous supply of external energy.
ΔG reaction 1300 2982 02165 ΔG reaction 1300 6456 12354 kJ mol -1. Gibbs free energy is represented using the symbol and typically has units of. Gibbs free energy and spontaneity.
G H - T D S. Free Energy and Free Energy Change the Gibbs free energy G is used to describe the spontaneity of a process. If enthalpy increases entropy thats going to be a spontaneous reaction.
Gibbs free energy and Temperature. ATP can release Gibbs free energy in packets of 305 kJ for each ATP converted to ADP. It is defined by the Gibbs equation.
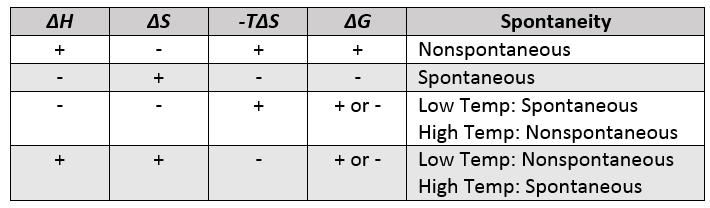
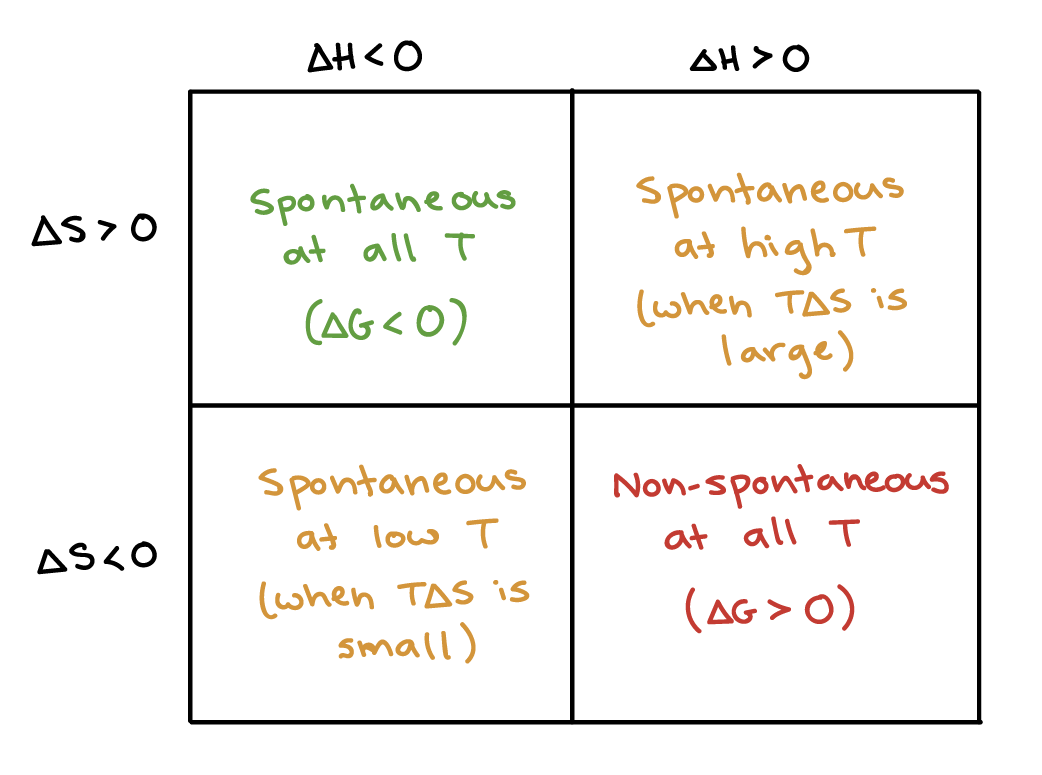
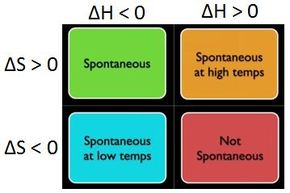
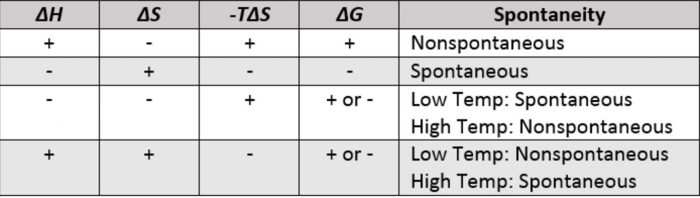
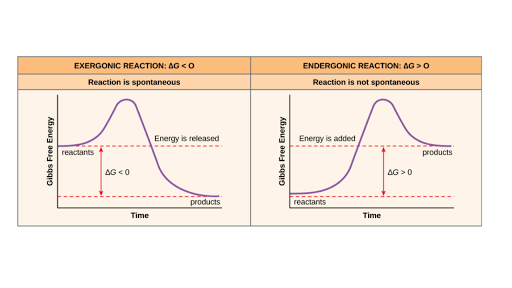
ΔG ΔH - TΔS In simple terms Gibbs Free Energy calculates the amount of energy available in a system to do work. Spontaneous Exergonic reaction. D G D H - T D S.
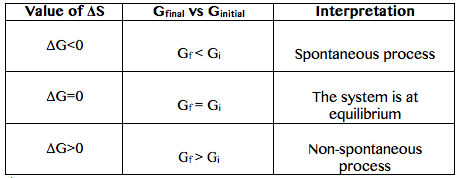
The easiest way to understand this situation while solving an equation is if G is negative then it is spontaneous. ATP H 2Ol ADP H PO4 aq. Graphing Free Energy as a Function Upon inspection the equation ΔG reaction ΔH reaction - TΔS reaction can be proven to represent a linear function where ΔG reaction is calculated over a series of temperatures while H reaction and ΔS reaction remain constant.
The transformation of a system from one state to another at constant temperature and pressure is spontaneous if the Gibbs free energy. Since we know that the change in the Gibbs free energy Δ r G o between products and reactants tells us whether or not the reaction will run spontaneously we will need this quantity at the new temperature. 2gare -1372 kJmole 876 kJmole and -3944 kJmole respectively.
If Δ r G o 0 the. G H - TSsystem For any spontaneous change G will be negative. This is given by the relationship.
ΔG 305kJmol Because ATP is high in Gibbs free energy it is said to be. When a process occurs at constant temperature and pressure we can rearrange the second law of thermodynamics and define a new quantity known as Gibbs free energy. ΔG ΔH - TΔS.
G can be ideally considered as standard free energy charge. Because this result agrees with the result we got when we calculated ΔG reaction using standard Gibbs free energy of formation values we are reasonably confident that our answer of 12352 kJ mol -1 is correct. This size is convenient for driving many biochemical processes in your body.